Editor's Choice, November 2016: how do you choose between organic growth, licensing or acquisition?
'Most early stage innovators need a deeper understanding of the models for taking a product to scale'. So says the introduction to this month's Editor's Choice. And this 2016 USAID report, Pathways to Scale: A guide for early-stage global health innovators on business models and partnership approaches to scale-up, provides a tool for them to gain exactly that. The focus is scaling medical products and technologies, but the clarity on business model options and decision-making tools, will be useful to others too.
Whereas our new Checklist on ingredients of scale focuses mainly on internal ingredients, such as mindset and margin, this guide focuses more on the particularly tricky issue of business models. As the introduction notes “many innovators 'struggle identifying the right pathway to scale for their innovation and ask themselves the same questions. Should I spin out a company to take my innovation forward on my own, or are there better-suited entities with more resources and capabilities to take it to scale faster? Should I partner, license out the technology, or give it away for free so that others with a similar interest can replicate and introduce the innovation more broadly? What are the key trade-offs and requirements I should consider, and when is the right time to make the change?”
If you or your grantees or investees face these questions, see if this guide can help.
The core of the report comprises three distinct segments. Five pathways to scale are outlined. Then nine case studies. And finally a Toolkit with three exercises.
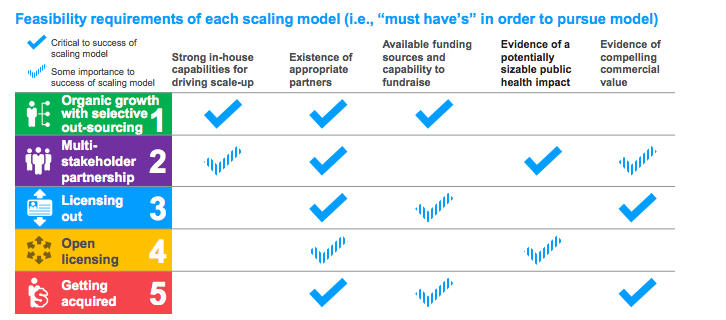
The five pathways are compared in terms of their pros and cons for the innovator and pre-requisites. This diagram compares the pre-requisites (page 6) while another similar diagram compares them in terms of implications for the entrepreneur, particularly their retention or loss of control.
The case studies show that in practice, it is rare for one pure model to be chosen and endure. The mixture of models used is well visualised. Examples include Sproxil Defender (pharmaceutical verification), 2 scaling pathways used for Shruti iHear (low-cost diagnostic of hearing problems), two neonatal devices (Laerdal NeoNatalie Resuscitator and D-Rev Brilliance phototherapy device) and Sushrut surgical implants. The pathways chosen and their reasons why are succinctly explained and clearly pictured. Each has achieved significant scale. For example, D-Rev Brilliance have installed close to 1,500 units that have impacted the lives of over 120,000 infants; and Shruti iHear has been used to screen more than 200,000 people so far.
The toolkit takes the entrepreneurs through an assessment of capabilities and resources, and based on that, identification of the best model to use. The final toolkit takes the entrepreneur through the key considerations for their chosen model.
A growing number of reports and events are talking about different models and pathways for scaling. There are some differing views on exact definitions, but broadly speaking alternatives are increasingly well known. Why I particularly like this report is that it goes so much further than simply classifying them. Delving into real studies, stylising them in terms of their scaling business model, and working through the implications of each model for the entrepreneur and for success, makes this a high value read.
Read the full report Pathways to Scale: A guide for early-stage global health innovators on business models and partnership approaches to scale-up
Read further editor's choice reviews of publications on key inclusive business topics
Further resources on scaling:
- Register for the Webinar on November 28th with William Davidson Institute & Hystra
- Gain insight on how to approach four critical scaling challenges and other tools and resources on the topic in our new know-how page on Scaling in BOP markets
This blog is part of the November 2016 series on Scaling and replicating inclusive business models, in partnership with DFID and SEED. Explore with us the key ingredients of a pathway to scale, debates and new ideas on replication, and look at what small companies, large companies and ecosystem actors can do.